SCIENCE
I. History
Streptococcal diseases have been known for centuries, although their delineation into separate disease entities did not begin to occur until the 16th century AD. The original writings of Hippocrates from the 4th century BC describe the disease erysipelas (red skin), as well as the symptoms of childbed fever, and “Galen remarks that not only erysipelas, but also inflammation, when it attacks the impregnated uterus, generally proves fatal”. Centuries later, after epidemic rates of mortality, this particular disease was recognized in 1716 as puerperal fever or childbed fever.
I. History
Scarlet Fever
Reviews of the writings of ancient scholars found many passages relating to sore throats or ulcerous tonsils, but lacked comment about an associated rash, which would be typical of what would later be known as scarlet fever. Giovanni Filippo Ingrassias, a Sicilian anatomist and practitioner, wrote in 1553 the first description of a disease termed “rossalia” that was characterized by “numerous spots, large and small, fiery and red, of universal distribution, so that the whole body appeared to be on fire.” Johann Weyer of the Netherlands was the first to describe a sore throat occurring during epidemics of scarlatina anginosa, which he did in 1565. In 1578, Jean Cottyar of Poitiers gave the first definitive description of scarlet fever in France as a “general weariness, headache, redness of the eyes, sore throat, and fever. Purpura appeared on the second or third day, accompanied by delirium and soreness of throat”. Daniel Sennert (1628) described an epidemic in Wittenberg in the beginning of the seventeenth century and was the first to describe scarlatinal desquamation, arthritis, and post-scarlatinal dropsy and ascites. The term “scarlatina” was first introduced into the medical literature in 1675 by Sydenham, who identified it as a separate disease entity from other exanthemas, especially measles.
Epidemics of scarlet fever were reported throughout Europe and North America during the 17th and 18th centuries, some of which were associated with high mortalities. It was not until the 1920s that George and Gladys Dick showed that scarlet fever was associated with a sore throat caused by hemolytic streptococci that produced a secreted toxin known as scarlet fever toxin, or Dick toxin.
I. History
Puerperal Fever
In 1843, the American surgeon Oliver Wendel Holmes, Sr. published a paper on “The Contagiousness of Puerperal fever”. He wrote, “This disease seized such women only as they were visited or delivered by a practitioner, or taken care of by a nurse, who had previously attended patients affected with the disease”. His observations were the subject of more debate than application, and many of his contemporaries responded to his report with more ridicule than acceptance.

In 1842, Ignac Semmelweis, a young Hungarian physician working in the obstetric wards of the Vienna Lying-in Hospital made the association between puerperal fever and soiled bed sheets, hygiene of medical doctors and midwives. He showed a dramatic reduction in disease when he asked medical doctors to wash their hands and use of chlorine solution to wash bedsheets. A full recognition of Semmelweis’s brilliant work did not come until 14 years after his death in 1865. In a discussion on puerperal fever at the French Academy of Sciences in Paris in 1879, a physician named Hervieux elaborated on the causes of epidemics in lying-in hospitals, ascribing them to an undefined “puerperal miasma. Louis Pasteur interrupted him: ‘None of those things cause the epidemic; it is the nursing and medical staff who carry the microbe from an infected woman to a healthy one.’ And when the speaker replied that he feared the microbe would never be found, Pasteur went to the blackboard and drew a diagram of the dangerous chain-forming microbe, saying, ‘There: This is what it looks like’.
I. History
The Early Recognition of Streptococci as Cause of Disease
The very first description of GAS in 1874 was made by an Austrian surgeon, Theodor Billroth, when he discovered the organism in cases of erysipelas and wound infections. In 1879, Louis Pasteur isolated the microorganism from the uterus and blood of women with post-labor uterine infections known as puerperal fever. He further demonstrated that the streptococcus was the etiological agent responsible for the disease that caused the highest mortality rates of women and newborns at that time.
In 1884, another surgeon, Fredrich Julius Rosenbach found streptococci in the pus of wound infections, which he named Streptococcus pyogenes. Rosenbach was certain that streptococcus was not a single species, but a genus. A striking feature of the Streptococcus, Rosenbach noted, was its ability to spread through extensive tracts of the host tissues, and live in them, without destroying the tissues or causing suppuration.
II. Group A Streptococcus (GAS)
A Group A Streptococcus (GAS) bacterial infection of the throat and skin, without treatment, can result in permanent damage to the heart.
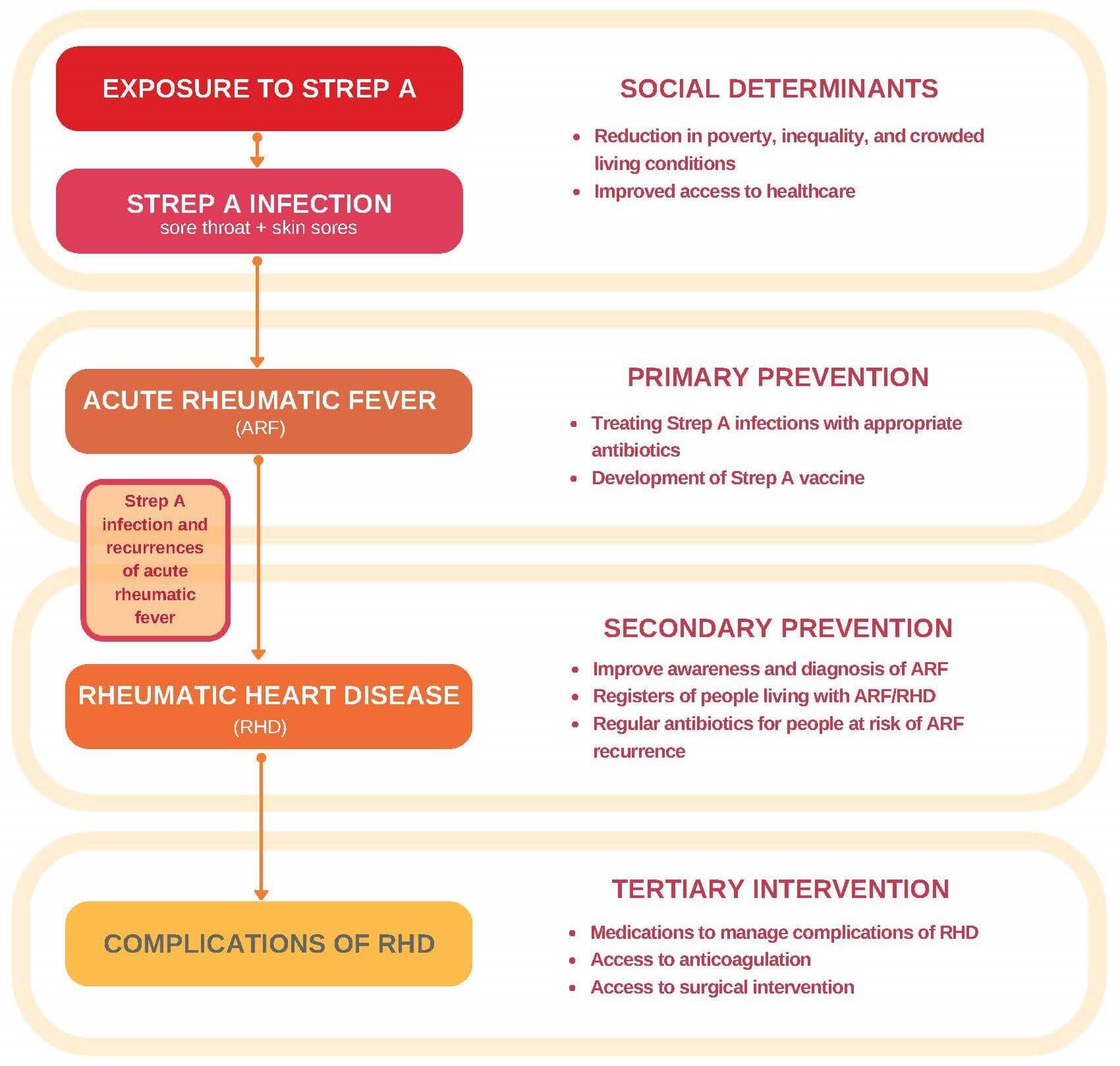
When a person gets a group A streptococcal infection of the throat (known as strep throat), their body’s immune system, in trying to fight that infection, produces antibodies. In severe cases, these antibodies, in addition to killing the strep, can damage their heart. Acute rheumatic fever can occur following an untreated strep throat infection and can cause irreparable damage to the major cardiac valves, known as rheumatic heart disease.
III. Global Disease Burden of Strep A
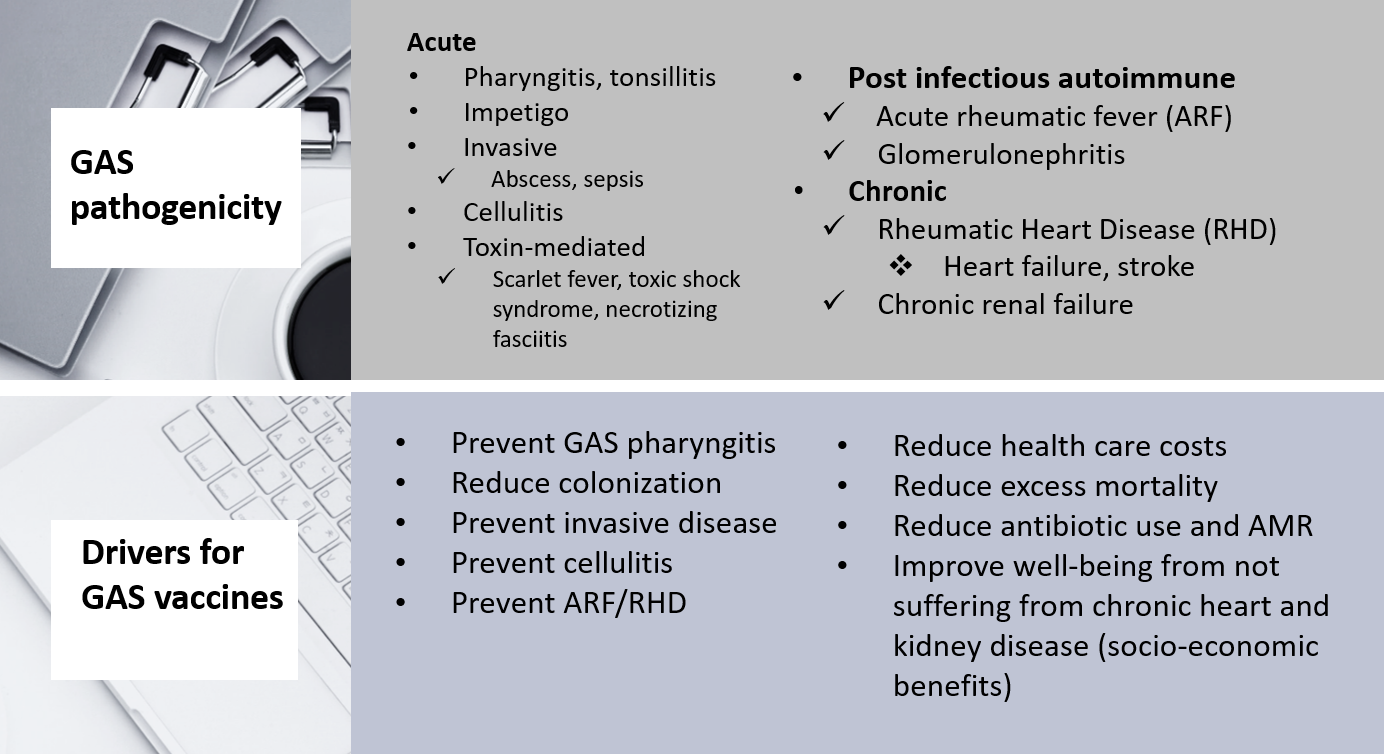
Despite being in existence for hundreds of years, Streptococcus pyogenes (group A streptococci) remains a significant cause of global morbidity and mortality, with a particular impact in resource-limited settings. The vast majority of cases of acute rheumatic fever (ARF), rheumatic heart disease (RHD), acute post-streptococcal glomerulonephritis (APSGN), and invasive S. pyogenes cases occur in low-resource settings. However, overall disease burden estimates are difficult, mainly because of variations in case definitions, the scarcity of comprehensive disease registries, the reliance on passive surveillance systems, and the underreporting of both acute and chronic cases. This is true in all regions, and particularly in countries where S. pyogenes diseases occur most frequently.
III. Global Disease Burden of Strep A
Non-invasive Strep A infection is the reservoir for acute and invasive diseases, both of which can lead to long-term pathogenicity.
Several million cases of non-invasive group A strep illnesses occur each year including pharyngitis, tonsillitis, skin infection (impetigo). It is roughly estimated that there are more than 111 million prevalent cases of GAS pyoderma, and over 616 million incident cases per year of GAS pharyngitis.
There are at least 517,000 deaths each year due to severe GAS diseases. The prevalence of severe GAS disease is at least 18.1 million cases, with 1.78 million new cases each year. The burden of invasive GAS diseases is unexpectedly high, with at least 663,000 new cases and 163,000 deaths each year. Invasive Group A Strep disease can include:
- Cellulitis
- Necrotizing fasciitis
- Abscess, sepsis
- Pneumonia
- Scarlet Fever
- Streptococcal toxic shock syndrome
III. Global Disease Burden of Strep A
Strep A disease affects both HIC and LMIC
- Pharyngitis, tonsillitis and skin infection (impetigo often associated with scabies in resource-poor settings) are universal Strep A diseases
- Data from the USA and Europe show substantial incidence and mortality rates due to invasive Strep A infection, but few data are available on morbidity. It is likely that child sepsis deaths may be due to Strep A infection. (Nelson et al. CID 2016)
- In a study conducted in 11 European countries, the risk of infection was highest among the elderly,
and rates were higher in males than in females in most countries.
- Skin lesions/wounds were the most common predisposing factor. Skin and soft tissue were the most common foci of infection, with 32% of patients having cellulitis and 8% necrotizing fasciitis. The overall 7-day case fatality rate was 19%; it was 44% among patients who developed streptococcal toxic shock syndrome. (Lamagni et al. J Clin Microbiol 2008)
- Low socioeconomic status is a known risk factor for Strep A disease, particularly for RHD, in First Nations populations of HIC countries such as Canada, Australia, New Zealand. Data remain however limited to associate invasive Strep A infection with poverty in most HIC.
- Sepsis became the leading 'direct' cause of maternal deaths in 2008 in the UK, and Strep A was/is the leading cause of those sepsis deaths. (Acosta & Knight. Curr Opin Obstet Gynecol 2013)
Low- and middle-income countries (LMICs) are affected the most. The combined mortality associated with RHD and invasive infections is the fifth leading cause of infectious disease deaths behind HIV, tuberculosis, malaria and S. pneumoniae.
- Poverty and overcrowding. Low socioeconomic status is a known risk factor for Strep A disease, particularly for RHD, in many different settings and countries.
- Burden is high in Africa and South Asia, and in indigenous Pacific populations
- Women are affected the most. Two-thirds of those afflicted by RHD are women, and importantly, the complications of RHD have a profound and detrimental impact on pregnant women and their children.
The Global Burden of Disease (GBD) study estimated that RHD affects 39 million people worldwide (5 million higher than the upper estimate for people living with HIV in the world), with more than 319,400 deaths per year. The GBD analysis did not separately account for invasive than Strep A disease, but it is conservatively estimated that invasive disease results in at least additional 160,000 deaths per year. RHD contributes to over 10 million disability-adjusted life-years (DALYs).
III. Global Disease Burden of Strep A
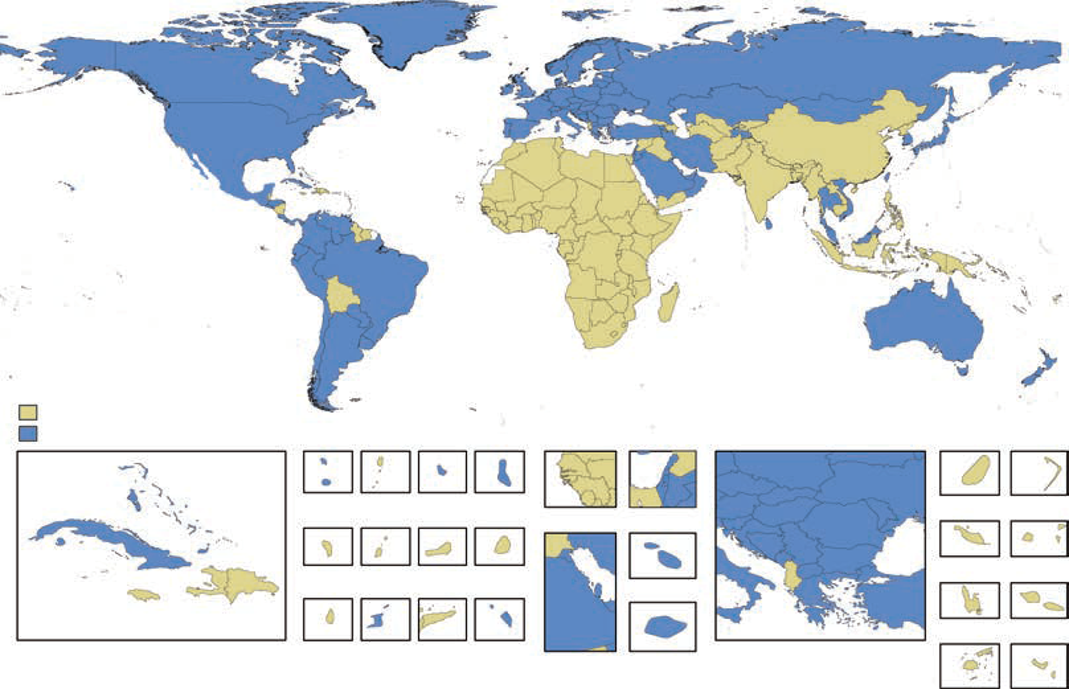
Classification of Countries as Having an Endemic or Nonendemic Pattern of Rheumatic Heart Disease.
A country was classified as having an endemic pattern of disease if its estimated childhood mortality due to rheumatic heart disease was greater than 0.15 deaths per 100,000 population among children 5 to 9 years of age. Watkins et al. NEJM 2017
III. Global Disease Burden of Strep A
Group A streptococcus (GAS) is the world’s most neglected cause of death from infection
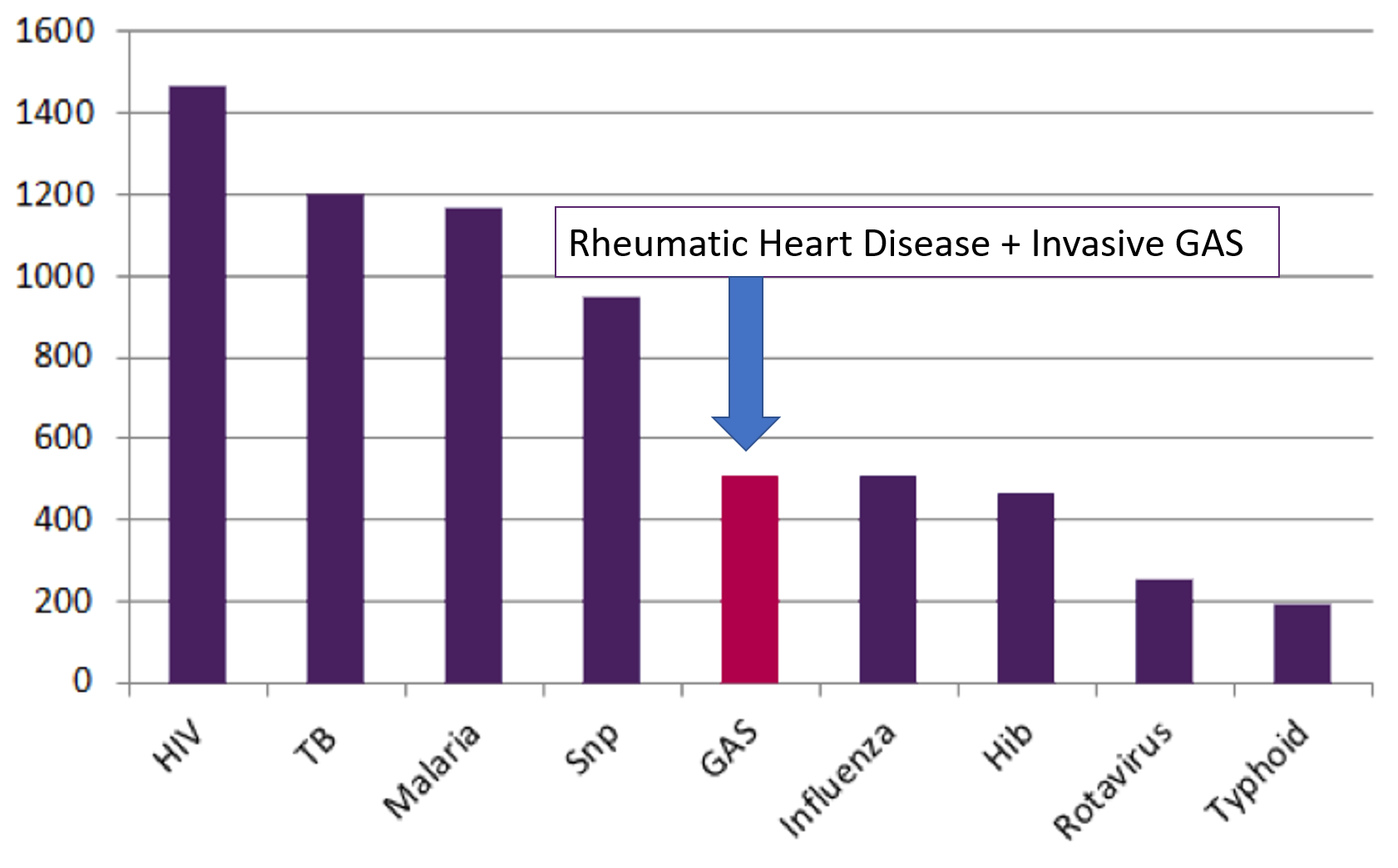
Lancet 2015;385:117-71; Lancet Infect Dis 2005;5:685-94
III. Global Disease Burden of Strep A
The global GAS population structure reveals extensive genomic heterogeneity
The M protein, encoded by the emm gene, is a major virulence factor and vaccine candidate and forms the basis of a number of classification systems. emm sequence typing is the most widely used method for defining group A streptococcal (GAS) strains, and has been applied to isolates in all regions of the world. The epidemiology of GAS disease in Africa and the Pacific region seems to be different from that in other regions, particularly high-income countries. In Africa and the Pacific, there were no dominant emm types, a higher diversity of emm types, and many of the common emm types in other parts of the world were less common. On the basis of the available data, the current formulation of the experimental multivalent emm vaccine would provide good coverage in high-income countries, particularly USA, Canada, and Europe, but poor coverage in Africa and the Pacific, and only average coverage in Asia and the Middle East.
More than 230 different M proteins have been described worldwide, potentially explaining variable capacity to induce rheumatic fever. Historically, a group of ten emm types have been epidemiologically associated with rheumatic fever (so-called classic rheumatogenic emm types). However, recent studies suggest that the concept of rheumatogenicity should be extended to include strains other than those classically described. Strep A vaccine candidate should offer broad coverage against a variety of genetic variants in order to protect against this serious sequela.
III. Global Disease Burden of Strep A
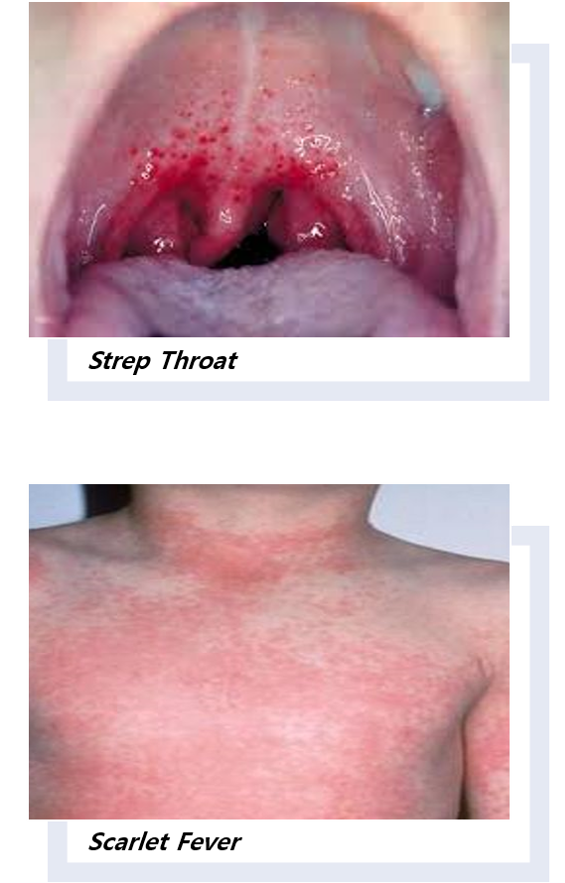
Pharyngitis, Skin Infection, and Scarlet Fever
GAS is the most common bacterial cause of acute pharyngitis. Over 616 million incident cases of GAS pharyngitis are estimated to occur globally each year, making this one of the most prevalent bacterial diseases in humans. Transmission primarily occurs through direct human-to-human contact, via respiratory droplets or nasal secretions from infected individuals. Illness predominantly occurs in children between 5 and 15 years of age but can also occur in adults. Disease severity of GAS pharyngitis is variable, but in most cases, it is a mild, self-limiting disease that resolves within 7 days. Characteristic symptoms include sudden onset of sore throat, fever, headache, abdominal pain, nausea, and vomiting. GAS can also asymptomatically infect the oropharynx for weeks to months at a time, during which time, little or no immune response to streptococcal antigens is elicited.
Occasionally, GAS pharyngitis is accompanied by scarlet fever, which is thought to result from pharyngeal infection with a GAS strain that secretes bacteriophage-encoded streptococcal pyrogenic exotoxins. Also known as scarlatina, scarlet fever manifests as a deep red, finely papular, erythematous rash; “strawberry tongue”; and exudative pharyngitis. While scarlet fever was a significant cause of childhood morbidity and mortality in the 19th and early 20th centuries, global rates have steadily declined over the last 150 to200 years such that it was considered a relatively rare disease until recently. However, recent outbreaks of scarlet fever in various parts of the world illustrate that scarlet fever remains a significant health problem.

GAS is one of the most important bacterial causes of skin and soft tissue infections worldwide. Specifically, it causes infections in the superficial keratin layer (impetigo) and the superficial epidermis (erysipelas). Impetigo is often associated with scabies, in particular in LMIC. The disease is spread through direct skin contact and most commonly affects children living in tropical and subtropical climates in areas with poor hygiene and crowded living conditions. The annual global disease burden is estimated to be 111 million cases. There is growing evidence regarding the potential importance of GAS skin infections as a cause of ARF, either alone or in combination with GAS pharyngitis.
III. Global Disease Burden of Strep A
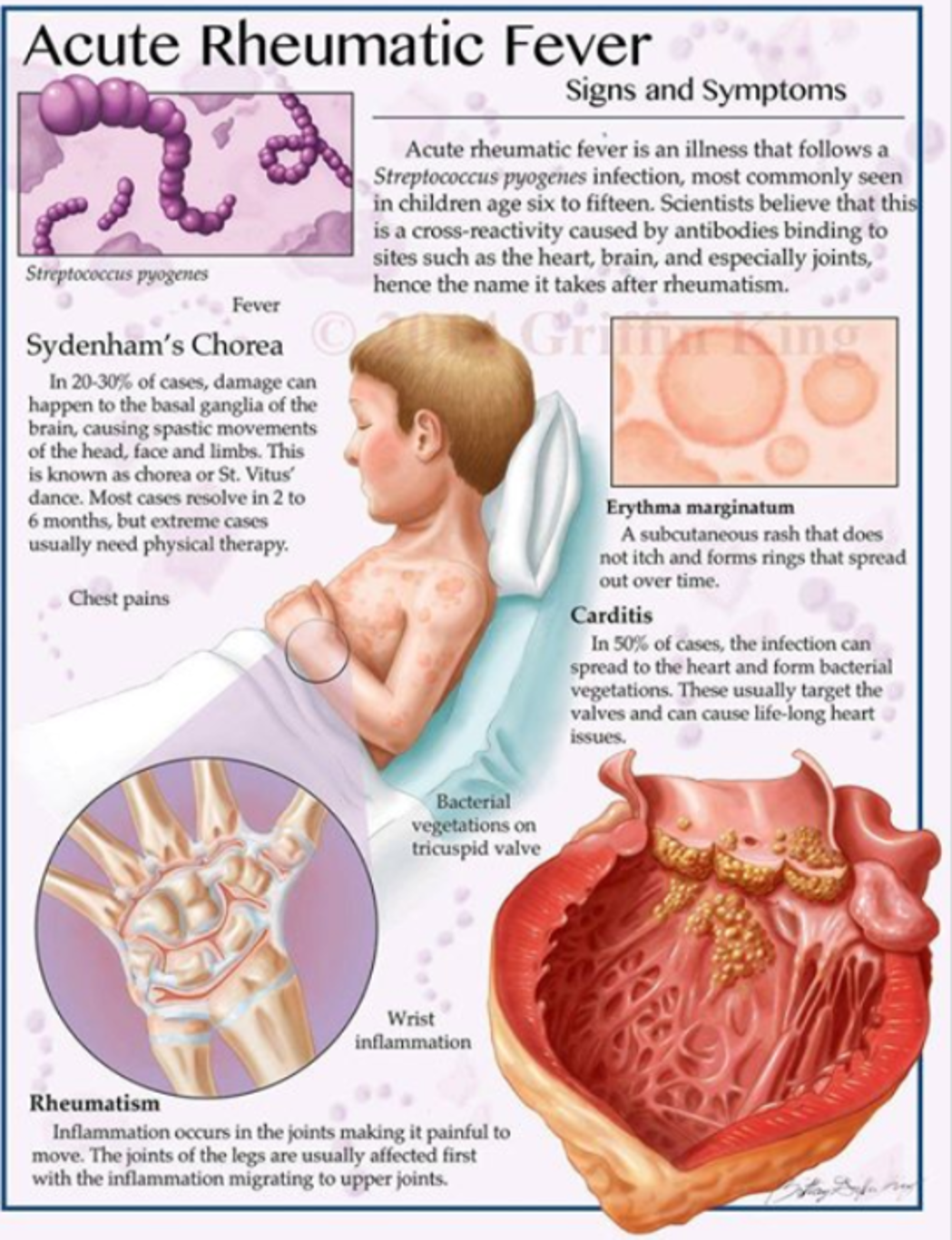
How Acute Rheumatic Fever and Rheumatic Heart Disease May Develop
Acute rheumatic fever (ARF) is the result of an auto¬-immune response to pharyngitis caused by GAS infection. ARF leads to an illness that is characterized by various combinations of joint pain and swelling, cardiac valvular regurgitation with the potential for secondary heart failure, chorea, skin and subcutaneous manifestations and fever.
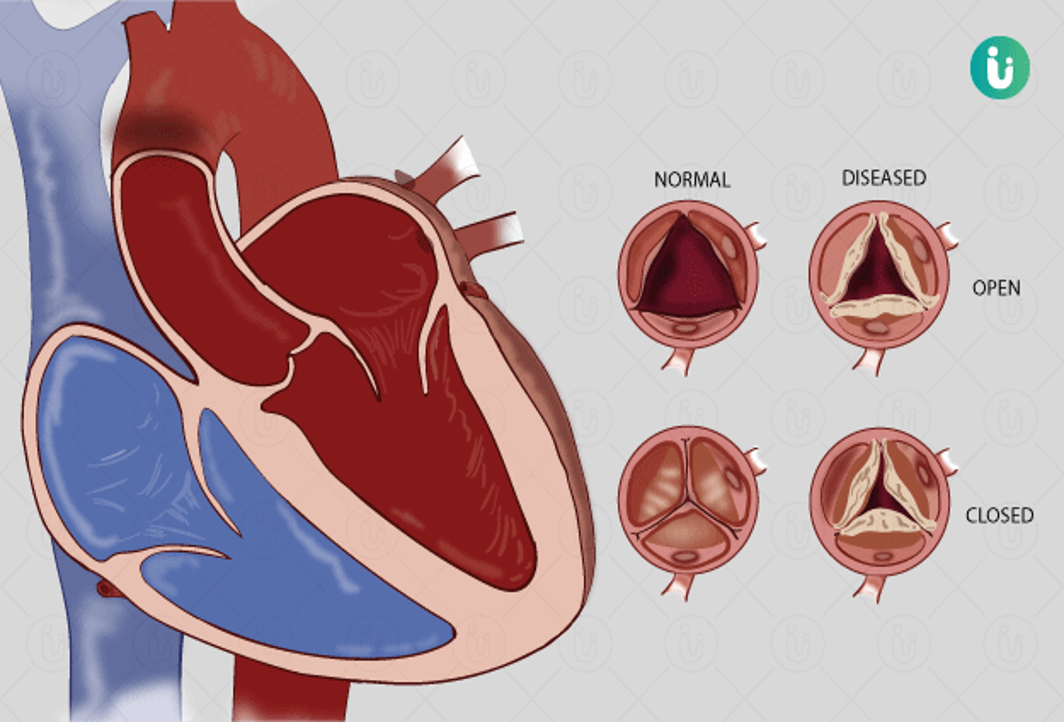
Rheumatic Heart Disease (RHD) corresponds to the persistent damage to heart valves resulting in mitral and/or aortic regurgitation, or in long-standing cases stenosis, that remains as a result of acute rheumatic fever with rheumatic carditis (active inflammation of the heart tissues, most importantly the mitral and/or the aortic valves). Complications of rheumatic heart disease include heart failure, embolic stroke, endocarditis and atrial fibrillation.
Most common clinical presentations of ARF*
Large joint arthritis and/or arthralgia, usually with fever, and sometimes with pansystolic murmur of mitral regurgitation Acute fever, tiredness and breathlessness from cardiac failure, with or without other manifestations (most commonly joint pain and/or swelling) and pansystolic murmur of mitral regurgitation Choreiform movements, commonly with behavioral disturbance but often without other manifestations Gradual onset of tiredness and breathlessness, which is indicative of cardiac failure, without fever or other manifestations, and pansystolic murmur of mitral regurgitation, which indicates the insidious onset of carditis
*Skin manifestations (erythema marginatum and subcutaneous nodules) are less commonly observed in ARF.
The relationship between GAS, ARF and RHD is critical and causal. Infection with GAS, typically untreated GAS pharyngitis or potentially skin infection, is followed weeks later by the presenting signs and symptoms of ARF, the case definition of which was recently revised to include echocardiographic criteria to improve the diagnostic sensitivity of the 1992 revised Jones criteria. More than 60% of individuals have evidence of RHD with the initial episode of ARF. Prolonged penicillin prophylaxis is effective in preventing recurrent attacks of ARF which has long been known to delay progression of RHD. However, in resource-limited settings appropriate penicillin therapy is not available and recurrent bouts of ARF following GAS infections are associated with progressive valvular involvement, most notably of the mitral and aortic valves. Echocardiography has revealed that valvular lesions consistent with RHD are found with high prevalence in LMIC.
RHD is one of the leading cardiovascular causes of DALYs lost in the <25-year age group. The impact is disproportionately distributed in LMIC. RHD accounts for 23% of strokes in Asia. Annual death rates in persons with RHD are estimated to be 6% in Pakistan and 12% in Ethiopia. Despite large gaps in data, underestimation of disease burden due to the difficulty in ascertaining cases of RHD seems likely. Thus, the current estimates of global impact may understate the problem, especially related to mortality. Some of the most affected countries have created the Global Rheumatic Heart Disease Registry (REMEDY study). This network may be extremely important to maintain and expand to monitor the impact of interventions and control of the disease. The findings of the REMEDY study (Zuhlke et al. European Heart Journal 2015; Circulation 2016), conducted in 14 LMIC, indicate that patients with clinical RHD, predominantly females, young, have high mortality and morbidity despite being young; those from low- and lower-middle–income countries had a poorer prognosis associated with advanced disease and low education. Twenty-two percent of persons already had atrial fibrillation, one-third had congestive heart failure, and most had no secondary prophylaxis with penicillin. Similar issues attend the use of anticoagulants in persons with atrial fibrillation and valve repair/replacement is not an option.
During pregnancy a marked increase in circulating blood volume is accompanied by a 30- 50% increase in cardiac output, which in addition to concurrent RHD can precipitate cardiac decompensation. Pregnant women with RHD are at risk of worsening cardiac status due to congestive heart failure. In women with symptomatic mitral stenosis, mortality may approach 50%, much of it postpartum. The progressive damage to heart valves caused by RHD is exacerbated by pregnancy. RHD may accounts for almost 90% of all heart disorders in women of child-bearing age in LMIC. Worldwide, roughly 25% of maternal deaths are classified as indirect. In a study in South Africa, 41% of these indirect deaths were from cardiac origin, predominantly RHD (71-84%) and in a Senegal study of 50 pregnant women with heart disease, 46 had RHD, resulting in 17 maternal deaths (34%). With the important caveat that these data are extrapolated from causes of maternal mortality and estimates of RHD, between 7-13.5% of maternal deaths may be due to RHD. In addition, RHD in a mother is associated with poor pregnancy outcomes, including intrauterine growth retardation and prematurity. In Nepal, pregnant women with RHD had a 4% mortality, with 16% fetal and neonatal mortality. RHD is associated with deaths in pregnant women, their children, and causes progressive maternal morbidity and mortality after delivery.
III. Global Disease Burden of Strep A
Cellulitis and Necrotizing Fasciitis
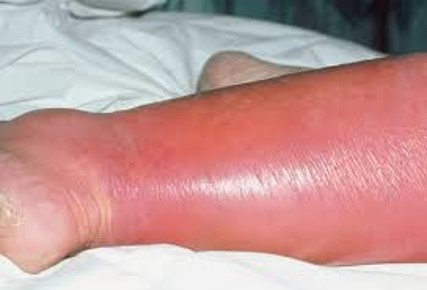
Cellulitis
Cellulitis is most commonly caused by streptococci bacteria, which enter the skin via a wound. The affected area is hot, tender, swollen and red, and there may be fever and chills.
Untreated cellulitis at the site of a wound may progress to bacteremia and septicemia or, occasionally, to gangrene. Cellulitis is usually more severe in people with reduced immune response, such as those with type II diabetes or an immunodeficiency disorder.
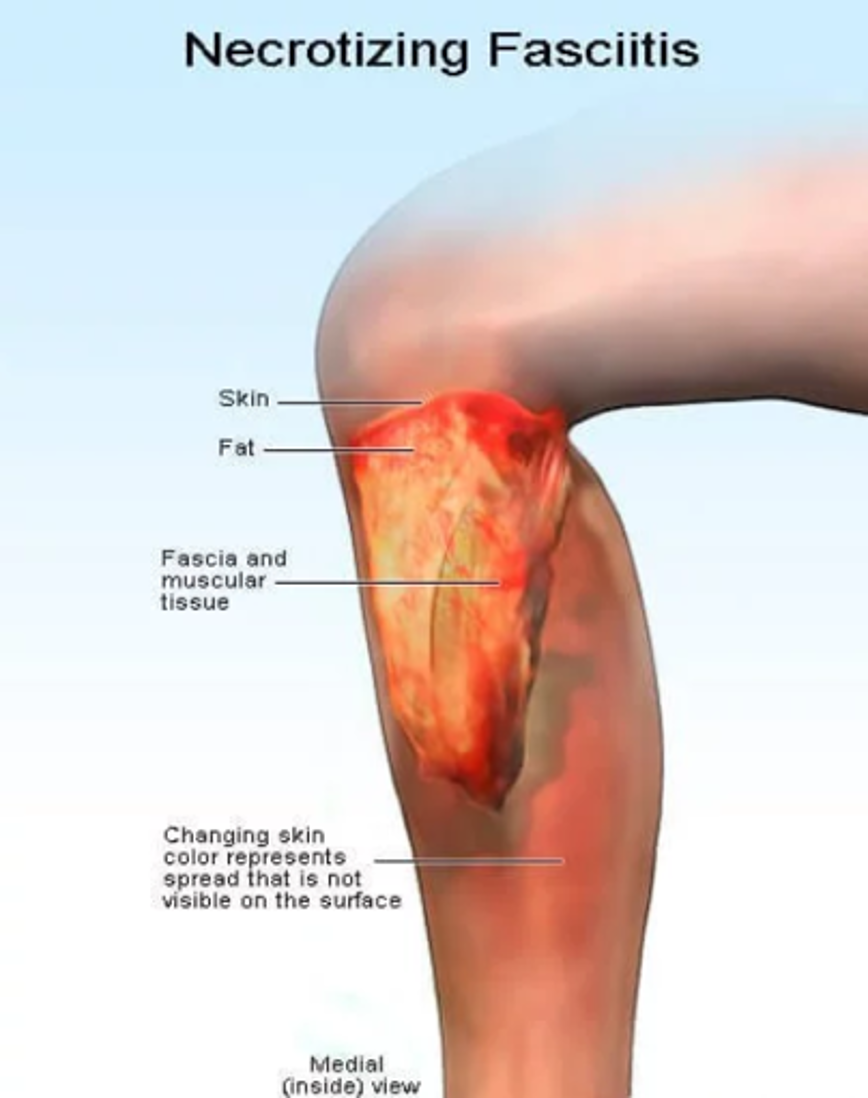
Cellulitis
Necrotizing fasciitis is a rare bacterial infection that spreads quickly in the body and can cause death. Although media reports often call it “flesh-eating bacteria,” more than one type of bacteria can cause necrotizing fasciitis. Public health experts believe group A Streptococcus are the most common cause of necrotizing fasciitis. Necrotizing fasciitis can be difficult to diagnose in the early stages as it can resemble cellulitis, which is a bacterial infection of the skin and the tissues beneath it.
In necrotizing fasciitis, the affected area is also hot, tender, swollen and red. There will also be fever and chills. These symptoms mirror those of cellulitis. However, the progressive changes of the skin will differ. Necrotizing fasciitis usually involves the formation of bullae (thin walled blisters), ulceration of the skin and black scabs. There is also heavy leakage of tissue fluid from the affected area and bubbles of gas in the tissues.
IV. Vaccines
Strep A kill half a million people a year. Why don’t we have a vaccine?

Substantial morbidity and mortality due to group A Streptococcus (GAS) diseases persists worldwide, yet development of safe and effective vaccines has been impeded by scientific, regulatory, and commercial obstacles. The majority of deaths from GAS occur in low- and middle-income countries (LMIC), most due to rheumatic heart disease (RHD). New cases of RHD are now rarely seen in high-income countries (HIC) outside of high-risk sub-groups. In settings endemic for RHD, even the most intensive control efforts have only demonstrated a partial effect. In addition to RHD, severe life-threatening invasive GAS infections occur worldwide, often presenting so acutely that there is no realistic window for prevention with early antibiotic treatment. More common milder syndromes including pharyngitis and skin infections cause significant social and economic disruption and costs.
Technically, development of GAS vaccines has been impeded by concerns that vaccine-induced responses might trigger autoimmunity and precipitate acute rheumatic fever, mimicking the relationship between GAS infection and the immunopathogenesis of acute rheumatic fever (ARF) and RHD.
The extensive strain diversity of GAS is a separate and additional technical challenge. From approximately 50 M serotypes, molecular typing of the M-protein hypervariable region has now described more than 220 emm genotypes, which have subsequently been grouped by genetic and functional similarities into smaller emm ‘clusters’. While the M serotypes, emm genotypes, and emm clusters are related, they are not fully-compatible classification systems. Sequencing of the hypervariable emm region and whole genome sequencing has more recently given insight into even greater levels of complexity and strain diversity. Several approaches have attempted to address these safety and efficacy concerns, avoiding the inclusion of antigens suspected to be responsible for autoimmunity and adopting either a multi-valent design or focusing on broadly conserved antigens.
There is no established correlate of natural or vaccine-induced protection against GAS infection in humans and very few longitudinal data from which putative correlates may be inferred. It is a longstanding and widely-propagated tenet that decreasing incidence of GAS pharyngitis with age is related to cumulative immunity following natural infections, although this does not offer lifelong protection and there is a spike in incidence of cellulitis and severe invasive GAS infections later in life.
A re-energized global vaccine development effort has organized around the vision of a safe, globally effective and affordable GAS vaccine to prevent acute GAS infections and associated antibiotic use, immune-mediated sequelae, and mortality. Its near-term aspiration is to demonstrate the safety and efficacy of a candidate vaccine against GAS pharyngitis and skin infections in children. Although RHD and severe invasive infections are the most fatal GAS syndromes, they are too uncommon and/or have too-long a latency period to be primary endpoints in clinical trials prior to licensure. Finally, demonstrating the value of a GAS vaccine to all stakeholders is crucial to attract the interest and investment from funders and industry required for development to proceed efficiently.
IV. Vaccines
First generation vaccines
Between 1833 and 1923, several empirical approaches were attempted to make a vaccine against scarlet fever. The first clinical trial of a GAS vaccine took place in 1923 following an earlier finding that asymptomatic carriage of GAS prevented symptomatic pharyngitis.
A number of killed whole-cell vaccines were studied, and then partially-purified M-protein vaccines, with mixed results and/or unacceptable reactogenicity. Parenteral or mucosal M-protein vaccines were administered to healthy volunteers who were subsequently challenged with homologous serotype strains by direct application to the pharynx using a swab. In 1968, a M3 protein vaccine candidate caused acute rheumatic fever in at least two children enrolled in a trial. The US Food and Drug Administration (FDA) responded in 1979 by issuing an embargo against further use of “uncontrolled, poorly defined preparations presumed to contain antigens that have been demonstrated in earlier studies to produce local and systemic reactions.” While the 2006 revocation of this ruling suggests the intention was that this would not apply to highly-purified well-characterized GAS antigens without demonstrable cross-reactivity with human tissues, the ruling had the practical effect of a ban. By the time the FDA lifted the ban what has been missing is pharmaceutical investment.
IV. Vaccines
Contemporary vaccine approaches
While there has been a steady increase in the number of pre-clinical vaccines since 2005 there has been a frustrating lack of progress in human clinical trials, with only four products tested in phase 1 and one vaccine reaching phase 2. None has entered efficacy trials and major vaccine industry players have largely remained on the sidelines.
Vaccine development for Strep A has primarily focused on the major cell surface protein, called M protein.
Strep A strains utilize M proteins for adherence to and internalization into epithelial cells by binding to various human proteins including fibrinogen, albumin, plasminogen and immunoglobin.
The M protein, encoded by the emm gene, is a coiled-coil protein composed of a variable N-terminal domain, a central region and a conserved C-terminal domain (see figure).
More than 230 different emm types of Strep A have been identified based on nucleotide variations in the N-terminal domain, giving rise to the serological specificity of the pathogen.
Antibodies against the M protein are opsonic by promoting phagocytic killing of the pathogen, thereby serving as a promising antigen for vaccine development.
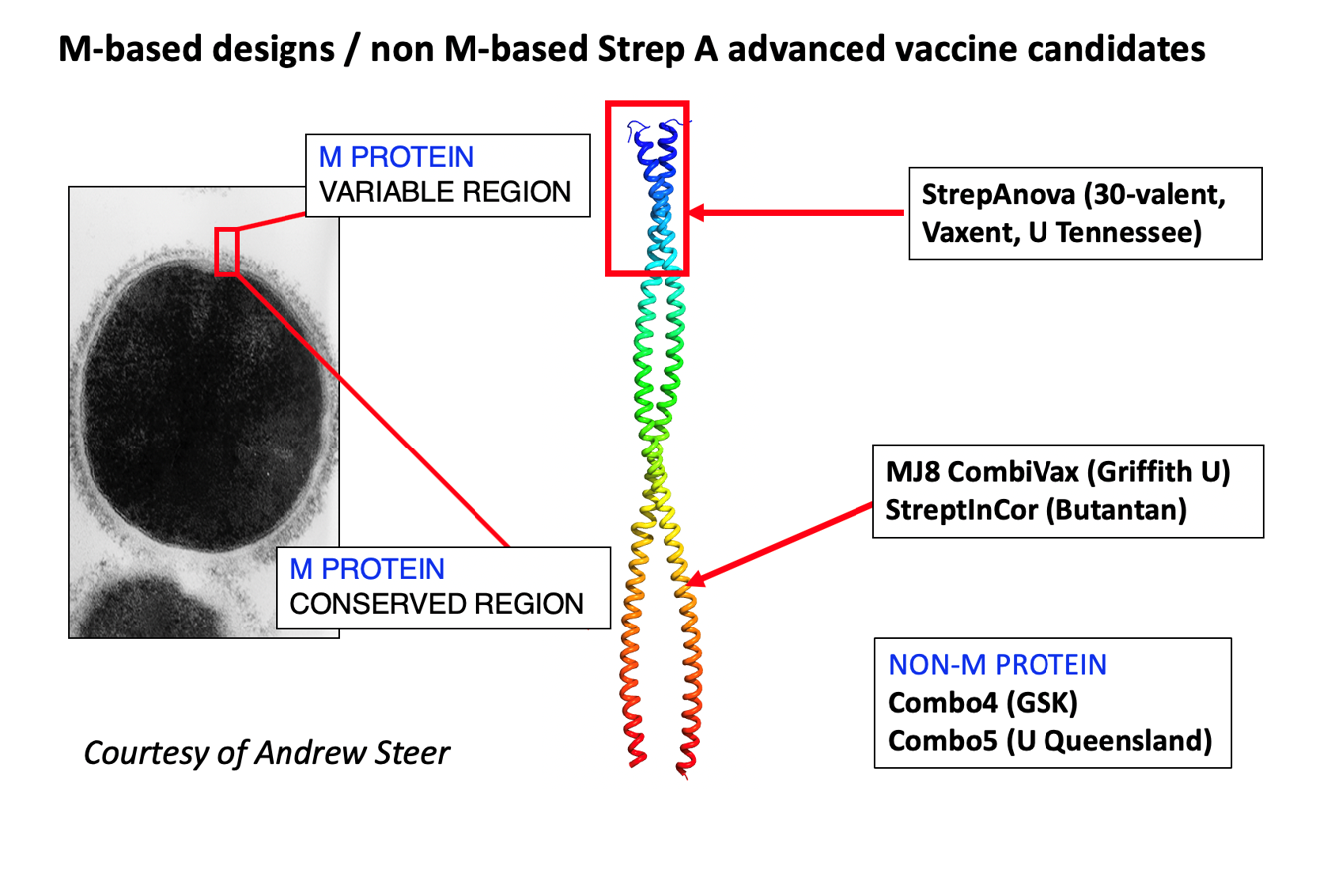
The most advanced strategy for an M protein-based Strep A vaccine has been the incorporation of multi-epitopic vaccines that protect against multiple strains.
The two major approaches to M-protein vaccine candidates include multivalent polypeptide N-terminal vaccines (30-valent, type-specific hypervariable region), and relatively conserved epitopes from the C-repeat region (minimal B-cell epitope derivatives of the p145 peptide, and a synthetic peptide vaccine comprising B- and T-cell epitopes derived from the C-repeat region of a M5 GAS strain). For both approaches, there have been careful steps taken to allay concerns of vaccine-induced immunity by avoiding cross-reactive epitopes.
Increasing interest in non-M-protein antigens has led from a desire to avoid GAS vaccine targets possibly implicated in autoimmunity. Significant immune responses do occur to a number of non-M-protein antigens following natural GAS infection in humans, and adult humans show increased responses compared to children suggesting that immunity to these relatively conserved antigens builds through repeated exposure. However, the contribution of these responses to protection against GAS infection in humans is unknown. The current leading non-M-protein candidate vaccine is the multiple-antigen ‘Combo’ vaccine, under development by GSK Vaccines Institute for Global Health. It should soon enter clinical development.
IV. Vaccines
New impetus for Strep A vaccine development

Stakeholder meetings in 2016 (Seoul) and 2018 (London) supported by the WHO Initiative for Vaccine Research (IVR), International Vaccine Institute, and the Wellcome Trust have led to the development and publication of WHO Preferred Product Characteristics and a Research and Development Roadmap for GAS vaccines in 2018, and formation of a Strep A Vaccine Global Consortium (SAVAC) in 2019. Identified as a priority activity in the WHO roadmap, the Development of a new controlled human infection model of GAS pharyngitis in healthy adults will offer a platform for early proof-of-concept studies for vaccines, building the confidence of research groups, industry, funders, and regulators to pursue later phase development. The WHO Product Development for Vaccines Advisory Committee (PDVAC) identified development of a Strep A vaccine as a priority.

The 71st World Health Assembly resolution report (12 April 2018) by the Director General of the World Health Organization (WHO) calls for action against RHD, including the development of a Strep A vaccine.
Together with national and regional initiatives, including the Australian and New Zealand Coalition to Advance Vaccines Against Group A Streptococcus (CANVAS) and a major Australian Medical Research Future Fund investment in 2019, these initiatives have built consensus and momentum towards a clinical vaccine development pathway focused initially on demonstrating efficacy against pharyngitis and skin infection, precursors to development of the immune-mediated sequelae ARF, RHD, and APSGN. Simultaneously, efforts are deployed to address critical and persistent gaps in knowledge, advocacy, and understanding the full public value of vaccines against GAS.
IV. Vaccines
Strep A and antibiotics
Except for macrolides, lincosamides, and streptogramins (MLS) and tetracyclines, S. pyogenes has remained highly susceptible to antimicrobial agents in vitro since the 1940s, particularly to penicillins, which are usually the first-line treatment. Indeed, even if therapy failures are quite common with β-lactams in clinical practice, no acquired mechanism of resistance has been reported to date. In cases of allergy or therapy failure, MLS antibiotics are considered to be alternate options. However, macrolide resistance may become a problem, since it has emerged in numerous countries. Besides MLS antibiotics, S. pyogenes can also acquire resistance to the tetracycline family. Notably, numerous clinical isolates are co-resistant to MLS and tetracyclines, since both resistance determinants are borne by the same mobile genetic elements. High-level resistance to aminoglycosides or fluoroquinolones remains very uncommon, while there is no (or exceptional) resistance to other antibiotics. More specifically, no resistance has been described to date for newer molecules (such as linezolid, tigecycline, and daptomycin).
IV. Vaccines
Vaccination against Strep A would have the potential to reduce antimicrobial resistance (AMR) by reducing antibiotic use
Rising AMR is one of the greatest health challenges the world currently faces. The global number of annual deaths from AMR is estimated at a minimum of 700,000. A World Bank simulation projects that the global economy could lose as much as 3.8% of its annual gross domestic product by 2050 in a worst-case scenario. Among the knowledge gaps identified, a better understanding of the epidemiology of AMR and Strep A in LMIC is needed and requires funding.
Vaccination as a solution has been largely undervalued. Vaccines can counteract AMR through multiple pathways. While vaccination can directly reduce the incidence of resistant infections, it also reduces both appropriate and inappropriate use of antimicrobials by reducing overall disease incidence, including infections caused by susceptible pathogens. This reduced antimicrobial use further diminishes pressure toward resistance among bystander members of the normal human flora.
This latter mechanism is the strategy by which vaccination against Strep A could massively reduce prescription of antibiotics – sore throat is among the top 3 reasons worldwide for prescription of antibiotics. In the US alone, there are 20 million visits per year for sore throat, and antibiotics are prescribed for more than 50% of children and 70% of adults. Vaccination against Strep A could have substantial secondary benefits in slowing development of AMR related to antibiotic overuse.
V. References
Barnett TC, Bowen AC, Carapetis JR. The fall and rise of Group A Streptococcus diseases. Epidemiology and Infection 2019;147:e4, 1–6.
Bennett J, et al. Understanding group A streptococcal pharyngitis and skin infections as causes of rheumatic fever: protocol for a prospective disease incidence study. BMC Infectious Diseases 2019; 19(633):2-10.
Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–94.
Carapetis JR. The stark reality of rheumatic heart disease. European Heart Journal (2015) 36, 1070–1073. doi:10.1093/eurheartj/ehu507
Carapetis et al. Acute rheumatic fever and rheumatic heard disease. Nature Reviews 2016;2:1-24.
Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016.
Osowicki J, et al. Controlled human infection for vaccination against Streptococcus pyogenes (CHIVAS): Establishing a group A Streptococcus pharyngitis human infection study. Vaccine 2019;37:3485–94.
Osowicki J, Vekemans J, Guilherme L, Steer AC, Kim JH. Group A Streptococcus Vaccines.Book chapter 14. In "Rheumatic Fever and Rheumatic Heart Disease” 2020. https://doi.org/10.1016/B978-0-323-63982-8.00014-3.
Schodel F, Moreland NJ, Wittes JT, et al. Clinical development strategy for a candidate group A streptococcal vaccine. Vaccine 2017;35:2007-2014.
Steer AC, Carapetis JR, Dale JB, et al. Status of research and development of vaccines for Streptococcus pyogenes. Vaccine 2016;34:2953-8.
Vekemans J, Gouvea-Reis F, Kim JH, et al. The Path to Group A Streptococcus Vaccines: World Health Organization Research and Development Technology Roadmap and Preferred Product Characteristics. Clin Infect Dis 2019;69(5):877–83.
Walker MJ, Barnett TC, McArthur JD, et al. Disease Manifestations and Pathogenic Mechanisms of Group A Streptococcus. Clinical Microbiology Reviews 2014;27;264-301.
Watkins DA, Johnson CO, Colquhoun SM, et al. Global, Regional, and National Burden of Rheumatic Heart Disease, 1990–2015. N Engl J Med 2017;377:713-22.
Zuhlke L, Engel ME, Karthikeyan G, et al. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). European Heart Journal 2015;36:1115-22.
Zuhlke L, Karthikeyan G, Engel ME, et al. Clinical Outcomes in 3343 Children and Adults With Rheumatic Heart Disease From 14 Low- and Middle-Income Countries. Two-Year Follow-Up of the Global Rheumatic Heart Disease Registry (the REMEDY Study). Circulation 2016;134:1456-66.
