WHAT WE DO
Paving the Way for a StrepA Vaccine that Benefits the World – Strep A Vaccine Global Consortium
The Second SAVAC Stakeholders Meeting, 06 June 2022
Date: June 06, 2022
Type of Event: The Second Stakeholders Meeting
The second stakeholders meeting organized by the Strep A Vaccine Global Consortium (SAVAC) under the leadership of its Executive Committee, chaired by Dr. Jerome Kim, was held in Stockholm, Sweden on June 6, 2022. The meeting welcomed the participants from academia, industry, non-profit organizations, and funding agencies in-person and virtually.
SAVAC members shared each workstream’s latest progress and achievements and gaps identified. Presentations covered the Strep A burden of disease, immunity and correlates of protection, vaccine safety considerations, and the Full Value of Vaccine Assessment. The SAVAC work and comments and recommendations gathered from the participants are a catalyst for SAVAC 2.0, a logical and necessary continuation aiming at filling the gaps and translating into action the development of Strep A vaccines to ensure that safe, effective, and affordable vaccines will be available to people in need.
We are extremely grateful to the outstanding speakers and participants, and in particular to the Wellcome Trust’s generosity in funding SAVAC, who together contributed to the great success of this meeting.

The Second SAVAC Stakeholders Meeting, 06 June 2022
The First SAVAC Stakeholders Meeting, 11 March 2021
Date: March 11, 2021
Type of Event: The Stakeholders Meeting
The first SAVAC stakeholders meeting organized by the SAVAC Executive Committee (chaired by Dr. Jerome Kim, Director General of IVI, and co-chaired by Prof. Andrew Steer, Murdoch Children’s Research Institute) and the IVI Secretariat was held on March 11, 2021. The virtual meeting gathered more than a hundred participants from all over the world.
The objectives of the meeting were to review the SAVAC achievements and work in progress, discuss critical issues of Strep A vaccine development—which includes burden of disease, vaccine safety, correlates of protection, vaccine landscape, business case and investment case as components of a full value of vaccine assessment—seek advice and recommendations on next steps and increase awareness and interest of funders.
We are very grateful to the outstanding speakers and participants who contributed to the great success of the meeting, and particularly grateful to the Wellcome Trust’s generosity in funding SAVAC.
Click here to download Meeting Report.
Overview of the SAVAC Safety Working Group (SWG)
Safety is a key workstream of SAVAC. The Safety Working Group (SWG) will be critical to further defining guidance and recommendations about appropriate safety screening and monitoring during the Strep A vaccine development process.
Safety concerns relevant to all vaccines exist for Strep A vaccines including classical reactogenicity events; however, there are also particular concerns relating to the non-purulent complications of Strep A infection (acute rheumatic fever and glomerulonephritis) that are specific to Strep A vaccines. Following a clinical trial in the 1960’s of a partially purified M protein vaccine led to two of the vaccinated children developing definite rheumatic fever and one developing probable rheumatic fever. This led to an FDA prohibition of Strep A organisms and derivatives from Bacterial Vaccines and Bacterial Antigens with “No US Standard of Potency” for human use in 1979. This prohibition was lifted in 2005. Nonetheless, concerns persist regarding auto-immune disease being induced by Strep A vaccines. In the clinical trials conducted since 2005, there has been no evidence of such auto-immune disease.
As vaccines move towards trials in larger groups of adult volunteers, vaccination of the target population (children) and vaccination of high-risk groups (populations where rheumatic fever is endemic), there is a need for clear guidance about appropriate safety screening and monitoring targeting vaccine developers, clinicians, and public health stakeholders. It may hopefully benefit to regulatory agencies.
The main focus of the Safety Working Group (SWG) with relevant experts and stakeholders is to review available data and the current status and guidance for safety assessment for Strep A vaccines at the pre-clinical stage, and all phases of vaccine clinical trials, and to develop published guidance for safety assessment and monitoring for Strep A vaccines at all stages of development, and for controlled human infection studies. The core membership of the SWG was chosen to enable broad levels of expertise across the full spectrum of Strep A vaccine development including clinicians, scientists, and importantly regulators.

Prof. Edwin J. Asturias
Prof. Edwin J. Asturias Chair
University of Colorado School of Medicine, Aurora,
Colorado, USA
Edwin J. Asturias is a
Professor of Pediatrics at the University of Colorado School
of Medicine and a Professor of Epidemiology at the Colorado
School of Public Health, in Aurora, Colorado, and holds the
Jules Amer Chair in Community Pediatrics at Children’s
Hospital Colorado. He is member of the SAVAC Executive
Committee.

Prof. Andrew Steer
Prof. Andrew Steer Co-Chair
Murdoch Children's Research Institute, Melbourne, Australia
Andrew Steer is a leading world advocate for
Group A Strep (GAS) vaccines, leading work on the molecular
epidemiology of the disease, and recently has begun to
develop a human challenge model for GAS pharyngitis. He is
Co-Chair of the SAVAC Executive Committee.

Dr. Jean-Louis Excler
Dr. Jean-Louis Excler
International
Vaccine Institute, Seoul, Republic of Korea
Jean-Louis Excler is pediatrician and vaccinologist with 30
years of experience in vaccine development. He joined the
International Vaccine Institute, Seoul, Republic of Korea in
2015 as Head of Clinical Development and Regulatory and
since 2018 is Program Director, New Initiatives. He is the
Project Lead of SAVAC.

Dr. Alma Fulurija
Dr. Alma Fulurija
Telethon Kids
Institute, University of Western Australia, Perth
Alma Fulurija is Project Lead of Australian Strep A Vaccine
Initiative (ASAVI). She is an immunologist with expertise in
immunology, vaccines and immunotherapy and has a strong
interest in understanding of host-pathogen interactions,
host defence mechanisms and immunity to infectious diseases.

Dr. Beno Nyam Yakubu
Dr. Beno Nyam Yakubu
National
Agency for Food and Drug Administration and Control, Nigeria
Beno Nyam Yakubu is an experienced
Regulatory Specialist with a demonstrated history of working
in the pharmaceuticals industry. Skilled in Good Laboratory
Practice (GLP), Vaccines, Biotechnology, Regulatory
Requirements, and Regulatory Documentation. As Chief
Regulatory Officer, he brings the experience and perspective
of the National Regulatory Authority of Nigeria.

Jim Ackland
Jim Ackland
Global BioSolutions,
Melbourne, Australia
Jim Ackland is a
scientist with over 40 years of experience in the
manufacture, quality control and international registration
of bio-pharmaceutical products. He is Managing Director and
CEO Global BioSolutions, Bio-Pharmaceutical Development and
Regulatory Affairs Consultant.

Dr. Raj Long
Dr. Raj Long
Bill and Melinda Gates
Foundation, Seattle, USA
Raj Long is a
senior executive with over 20 years of experience in drug
development. Raj brings a unique strategic expertise on
regulatory affairs. She is currently a Deputy Director at
the Bill & Melinda Gates Foundation (BMGF).

Dr. Wellington Sun
Dr. Wellington Sun
Senior
Consultant, Vaxcellerant, Silver Spring, Maryland, USA
Wellington Sun is infectious disease
physician-scientist with diverse expertise in clinical
research and FDA regulation of infectious disease vaccines
and other biotherapeutics. He was Director of the Division
of Vaccines and Related Product Applications at the FDA for
10 years and is currently a vaccine consultant at
Vaxcellerant LLC.

Dr. Marco Cavaleri
Dr. Marco Cavaleri
European
Medicines Agency (EMA), Amsterdam The Netherlands
Marco Cavaleri is Head of Anti-infectives and Vaccines, EMA.
He has spent several years in industry in Research &
Development for antibacterial, TB and antifungal drugs,
including both non-clinical and clinical aspects. He has
been at EMA for nine years, first as scientific advisor for
vaccines and anti-infective agents, later as coordinator of
regulatory activities in these areas including 2009 pandemic
influenza scientific activities.

Dr. Adwoa Bentsi-Enchill
Dr. Adwoa Bentsi-Enchill
World
Health Organization, Geneva, Switzerland
Adwoa Bentsi-Enchill is an epidemiologist in the Department
of Immunization, Vaccines and Biologicals of the World
Health Organization, Geneva. Adwoa has over 25 years
expertise in public health with a focus on vaccine
preventable diseases. At WHO she has worked primarily on
invasive salmonelloses and immunization safety, and has had
significant experience collaborating with key global
stakeholders as well as providing technical support to
several countries across WHO’s six regions.
Program Management

Somyoung Cho
Somyoung Cho
International Vaccine
Institute, Seoul, Republic of Korea
Somyoung Cho is Project Manager in vaccine development and
delivery at IVI. She has 20 years of professional
experience with non-profit international organizations and
industry in Korea and in France. Somyoung is Project
Manager of SAVAC since 2019.

Matthew Parnaby
Matthew Parnaby
Murdoch Children's
Research Institute, Melbourne, Australia
Matthew Parnaby is a Public Health professional with an
emphasis on healthcare for vulnerable populations. He has
20 years of experience in Public/Primary Health Care in
Emergency/Development operations in the Middle East,
Africa, Eastern Europe, Central and South East Asia and
Australia. Matthew has had an operational focus in the
Tropical Diseases Group, facilitating and streamlining
research projects and programs in Australia and the Pacific
Region.
Overview of the Strep A BoDWG
The Strep A Burden of Disease Working Group (BoDWG) was first established in 2020 through the Strep A Global Vaccine Consortium (SAVAC). This group was initially comprised of 13 members from 7 geographically diverse countries representing 5 WHO regions with expertise in clinical medicine, epidemiology, surveillance, health economics and global vaccine policies covering Strep A and other vaccine-preventable diseases. The group had a commitment to advise SAVAC on the key burden of disease issues that need resolving to progress the prevention of Strep A through vaccination. With limited funding, the group has made important contributions to the success of SAVAC. This included the publication of a series of standardized Strep A surveillance protocols which established the framework methodology for surveillance activities within SAVAC 2.0, development and publication of a seminal “data purpose matrix” to guide prioritization of data for Strep A vaccine development, and development of a list of priority research projects focused on burden of disease. The purpose of the BoDWG is to build the evidence base around the Strep A burden of disease, with a particular focus on data that may accelerate development and implementation of Strep A vaccines. This will be done by establishing a community of practice around Strep A burden of disease and epidemiology global research as well as continuing to provide strategic advice to SAVAC 2.0, in particular Workstream 1: Preparing for vaccine trial sites. The BoDWG will also engage in new research activities as decided by the group. The Secretariat of the BoDWG and its organisation will reside within The Kids Research Institute Australia. Current members of the BoDWG are listed below:

Prof. Jonathan Carapetis
Prof. Jonathan Carapetis Co-Chair
The Kids Research Institute Australia
Prof. Jonathan Carapetis is the Director of The Kids
Research Institute Australia in Perth, Western Australia, an
infectious diseases consultant physician at the Perth
Children’s Hospital. Prof. Carapetis is a leader in the
study of Group A streptococcus epidemiology and disease
burden. He has long been an advocate for the development of
Strep A vaccines.

Dr. Chris Van Beneden
Dr. Chris Van Beneden Co-Chair
Centers for Disease Control and Prevention
Dr. Chris Van Beneden is a public health consultant. She
worked as a medical epidemiologist for the U.S. Centers for
Disease Control and Prevention (CDC)’s for over 20 years.
While at CDC, Dr. Van Beneden directed the epidemiologic
research, policy development and public health response
efforts pertaining to group A strep. She is an expert in
surveillance for community acquired bacterial infections,
respiratory outbreak management, and the study of vaccines
to prevent pneumococcal disease.

Prof. Michael Baker
Prof. Michael Baker
Professor Michael Baker is a public health physician,
epidemiologist, researcher, and science communicator based
in the Department of Public Health, University of Otago,
Wellington.
He has a long-term research focus
on rheumatic fever and Streptococcal disease which has
included surveillance, descriptive epidemiological studies,
aetiological studies (case-control, record linkage),
intervention evaluations, burden of disease analyses, and
research syntheses.
He leads the Health
Protection Aotearoa Research Centre (HPARC) which focuses on
infectious diseases and environmental health. The group has
active research projects in these areas, including rheumatic
fever, respiratory infections, and improving infectious
disease surveillance.
Michael took a leading
role in shaping New Zealand’s Covid-19 pandemic response,
particularly the elimination strategy. He was a member of
the Government’s Covid-19 Technical Advisory Group
throughout the pandemic.
Michael has a strong
interest in science communication and evidence translation
and is the inaugural director of the Public Health
Communications Centre Aotearoa (PHCC), which he established
in February 2023.

Dr. Andrea Beaton
Dr. Andrea Beaton
Andrea
Beaton is a Professor of Pediatrics and Pediatric
Cardiologist at Cincinnati Children's Hospital. She is a
founding member of the Rheumatic Heart Disease Research
Collaborative in Uganda (RRCUganda.org), where she
spearheads initiatives to combat rheumatic heart disease on
a global scale.
Dr. Beaton holds leadership
positions with the American Heart Association, the World
Heart Federation, and the World Health Organization, where
she serves as Co-Chair of the RHD Guideline Development
Group. Her work is supported by a remarkable network of
mentors and collaborators dedicated to the shared mission of
eliminating RHD. Dr. Beaton lives in the US with her four
children and her extraordinary husband, who inspire her
commitment to making the world a healthier and more
equitable place for all.

Prof. Asha Bowen OAM
Prof. Asha Bowen OAM
Professor Asha Bowen OAM is a clinician scientist working
across the Perth Children’s Hospital as a paediatric
infectious disease specialist and The Kids Research
Institute Australia (formerly Telethon Kids Institute) as
Head of the Healthy Skin and ARF Prevention Team.
Asha
has more than 15 years’ experience leading infectious
diseases research and investigator-initiated clinical trials
focused on issues significant to Aboriginal child health and
ARF prevention. Asha focuses on primordial and primary
prevention of Strep A related diseases through a
comprehensive healthy skin program and exploring new
opportunities for prevention through early diagnosis at the
point of care.

Dr. Jeffrey Cannon
Dr. Jeffrey Cannon
Dr
Jeffrey Cannon is a senior health economist and researcher
at The Kids Research Institute Australia. His research
focuses on understanding the interactions between health,
economic, and social values and developing and applying
analytic models to facilitate policy decisions.
He earned a PhD in Health Economics from the University of
Western Australia in 2019 and previously received an Honours
degree in Mathematics after a BSc (Mathematics) and BBus
(Finance) dual degree. Dr Cannon’s PhD thesis included the
first-ever estimates for the health and economic burdens of
all major group A Streptococcal (Strep A) clinical
manifestations and the potential value of vaccines in
Australia. Following his PhD, Dr Cannon took up a
post-doctoral position at the Harvard T.H. Chan School of
Public Health in Boston during 2019-2022, where he was a
scientific lead in developing a Full Value of Vaccines
Assessment for Strep A vaccination globally.

Dr. Thomas Cherian
Dr. Thomas Cherian
Dr Thomas
Cherian is a Managing Partner at MMGH Consulting, based in
Geneva, Switzerland. Before this, he worked at WHO in Geneva
for 17 years, where he served as the Coordinator for the
Expanded Programme on Immunization and before that as the
Coordinator for Implementation Research in the Initiative
for Vaccine Research. He also holds the position of Senior
Associate in the Department of International Health at the
Johns Hopkins Bloomberg School of Public Health, Baltimore,
USA.
Before joining WHO, Dr. Cherian was a
Professor of Paediatrics at the Christian Medical College in
Vellore, India, where he did his medical and postgraduate
training in paediatrics. Subsequently, he did a 3-year
fellowship in Paediatric Infectious Diseases at the Johns
Hopkins University School of Medicine, Baltimore.
Dr.
Cherian has authored or co-authored over 170 scientific
articles and book chapters. His main research interests have
been related to acute respiratory infections in children.
His research has contributed to the case management
protocols for acute respiratory infections in children and
to policy development for the use of Pneumococcal and Hib
vaccines worldwide. During his tenure as the EPI Coordinator
at WHO, he oversaw the rollout of new vaccines in low- and
middle-income countries and the measurement of their impact.
He established the WHO sentinel site surveillance network
for invasive bacterial infections and diarrhoeal diseases.
Through MMGH Consulting, Dr. Cherian continues to
support multi-national agencies, including WHO, UNICEF, and
Gavi with immunization policy, strategy, programme
implementation and evaluation.

Prof. Mark E Engel
Prof. Mark E Engel
Professor Mark E Engel is a researcher based in the SA
Cochrane Centre, SA Medical Research Council, South Africa,
where he serves as the unit director. Initially a medical
laboratory scientist, he was later a Harvard University
fellow in Public Health in 2001, before pursuing a career in
epidemiology. Following completion of his MPH degree, he was
awarded a PhD from UCT in 2013 for a large longitudinal
study on the epidemiology of RHD in Cape Town children.
Prof Engel networks with African colleagues to apply
a wide range of investigative approaches, from molecular to
population-based research to all aspects of Rheumatic Heart
Disease. In 2016, he established the AFROStrep Study, in
efforts to improve the understanding of the role of group A
streptococcus in RHD development and inform putative
vaccines under development.
His work,
supported over the years by the SA National Research
Foundation, the SA Medical Research Council, the American
Heart Association and NIH in the USA, has been presented
both nationally and internationally. He has an excellent
academic record, teaching health research methods and having
successfully supervised to completion a number of students
undertaking MPH, MSc and PhD degrees. Finally, Prof Engel is
a member of the African Union/Pan-African Societies of
Cardiology Education Taskforce and the Acute Rheumatic Fever
(ARC) Diagnostic Network.

Dr. Theresa Lamagni
Dr. Theresa Lamagni MSc PhD
HonMFPH
Dr Theresa Lamagni is a Section
Head Healthcare-Associated Infection & Antimicrobial
Resistance Division of the UK Health Security Agency and
designated epidemiologist for the WHO Collaborating Centre
for Streptococcal Diseases in London. She has worked in
public health for over 25 years. During this time she
completed an MSc in Epidemiology at the London School of
Hygiene & Tropical Medicine and a PhD on the epidemiology of
invasive GAS infections in Europe at the University of
Helsinki.
Theresa has strategic
responsibility for the national surveillance of
streptococcal diseases, providing expert support to outbreak
and incident response and contributing to the development of
an evidence base to inform disease prevention programmes.
She has authored over 180 peer-review papers, 4 clinical
microbiology book chapters and 4 national public health
guidelines and sits on the editorial board of the Journal of
Antimicrobial Chemotherapy.

A/Prof Hannah Moore OAM
A/Prof Hannah Moore OAM
A/Prof Hannah Moore OAM is an infectious disease
epidemiologist, Co-Head of the Infectious Disease
Epidemiology team within the Wesfarmers Centre of Vaccines
and Infectious Diseases at The Kids Research Institute
Australia and Associate Professor at the School of
Population Health, Curtin University in Western Australia.
Her passion for research involves using
population-based data to investigate how to recognise,
prevent and reduce serious respiratory and other infectious
diseases in children through estimating burden of disease
and evaluating the effectiveness of vaccination programs.
She has developed expertise in the epidemiology of
Respiratory Syncytial Virus (RSV) in young children, where
her research was pivotal in WA Governments’ decision to
establish the first and most comprehensive RSV infant
immunisation program in the nation. Raising awareness of
RSV, understanding community burden and evaluating the
impact of prevention measures is now a major focus of her
research program. She has previously contributed to state
and national influenza vaccination policy.
In
2020 A/Prof Moore joined the Strep A Vaccine Global
Consortium (SAVAC) to increase knowledge and awareness of
the global burden of Group A Streptococcal diseases. She now
holds an activity lead position in the US$11M-funded SAVAC
2.0.
A/Prof Moore has been awarded >$19M in
competitive research grants, co-authored >140 papers, was
TEDxPerth 2018 speaker, recipient of a WA Young Tall Poppy
Award (2013) and the WA Premiers Science Early Career
Scientist Award (2015). In 2024, she was honoured with a
Medal of the Order of Australia for her service to
epidemiology as a researcher.

A/Prof David Watkins
A/Prof David Watkins
David
Watkins is an Associate Professor in the Division of General
Internal Medicine and in the Department of Global Health at
the University of Washington. He studies health system
reform and policy implementation challenges, with a
particular emphasis on non-communicable diseases in low- and
middle-income countries. Much of his work uses rheumatic
heart disease as a tracer condition for non-communicable
diseases more broadly.
His research team works
in three thematic areas: (1) population and economic
modelling to support policy analysis, (2) integrated
healthcare delivery, and (3) use of evidence in policy
formulation. His work has been featured in several
high-impact journals, and has been an advisor to, or member
of, numerous international committees, task forces,
commissions, and working groups related to global health
policy and systems.
He also continues to
practice as an internal medicine specialist at Harborview
Medical Center in Seattle. He received a Bachelor of Science
from Rhodes College and a Doctor of Medicine from Duke
University before moving to Seattle, where he completed a
residency in internal medicine at the University of
Washington, an MPH through the Institute for Health Metrics
and Evaluation, and a research fellowship in health
economics with the Disease Control Priorities Network.
The 71st World Health Assembly Wraps Up With Adoption of Resolutions on Rheumatic Fever and Rheumatic Heart Disease
Date: 21–26 May 2018
Type of Event: Adoption of Resolutions
The 71st World Health Assembly, the decision-making body of the World Health Organization (WHO), was attended by delegations from all WHO Member States. The Assembly focused on a specific health agenda prepared by the Executive Board including Rheumatic Fever and Rheumatic Hearth Disease of which Group A Streptococcus (GAS) is the precipitating cause. The government of New Zealand led the drafting process to develop the Resolution, stated: ‘the facts and figures are clear.’ The delegation of Namibia also demonstrated that the number of people living with RHD around the world was comparable to those living with HIV. Member States of the WHO unanimously adopted a “Global Resolution on Rheumatic Fever and Rheumatic Heart Disease,” which was co-sponsored by countries from all six WHO regions.
Draft resolution proposed by Australia, Brazil, Canada, Cook Islands, Ecuador, Fiji, Japan, Namibia, New Zealand, Pakistan, Samoa, Tonga and Tuvalu, May 2017
Geneva, Switzerland, the 71st World Health Assembly, May 2018
IVI and WHO Host Global Consultation on Group A Streptococcal Vaccine Development
Date: December 12-13, 2018
Type of Event: WHO Consultation
The International Vaccine Institute (IVI) and the World Health Organization (WHO) co-hosted the Global Stakeholder Consultation on Group A Streptococcal (GAS) Vaccine Development at the Sheraton Seoul Palace Gangnam Hotel from December 12-13.
The one-and-a-half-day meeting convened international scientific experts, vaccine developers and funders to review evidence on GAS burden of disease and the need for a vaccine, and to discuss the feasibility and pathway for developing GAS vaccines. The meeting was in line with WHO’s goal to accelerate the development and licensure of high-quality, safe and effective GAS vaccines for low-and middle-income countries.
“The consultation provides an excellent opportunity to discuss, coordinate and mobilize resources and action at a global level to take concerted action against group A Streptococcus,” said Jerome Kim, IVI Director General, “The increasing body of evidence shows that the burden of GAS diseases and its linkage with poverty cannot be ignored. The global health community must work together to accelerate the development of a vaccine against this major killer.”

Seoul, Republic of Korea, International Vaccine Institute, December 12-13, 2016
The WHO Consultation on GAS Vaccine R&D
Date: March 16-17, 2018
Type of Event: WHO Consultation
In May 16-17, 2018, the WHO Consultation on GAS Vaccine R&D was held in London, UK. About 70 scientists from the whole world attended the consultation. The consultation was initiated by reviewing the present status of GAS Vaccine R&D and identifying bottlenecks. All the top brains of various Companies and Institutes that are interested in the GAS vaccine, gathered together, discussing and sharing the latest advances in the field.
The World Health Organization's Initiative for Vaccine Research (WHO IVR) aims to promote and accelerate the development of Streptococcus A vaccines, global health needs highlighted by the WHO PDVAC. Accordingly, WHO preferred product characteristics (PPC)—an early development stage precursor to class- or product-specific target product profiles— have been proposed and an R&D technology roadmap presented
The meeting took place at the Wellcome Trust Headquarters in London. The Wellcome Trust was a potential donor to accelerate Strep A vaccine development through the constitution of a consortium. IVI applied to lead this consortium and was awarded the 24-month Welcome Trust grant in 2019.


London, UK, Wellcome Trust, May 16-17, 2018
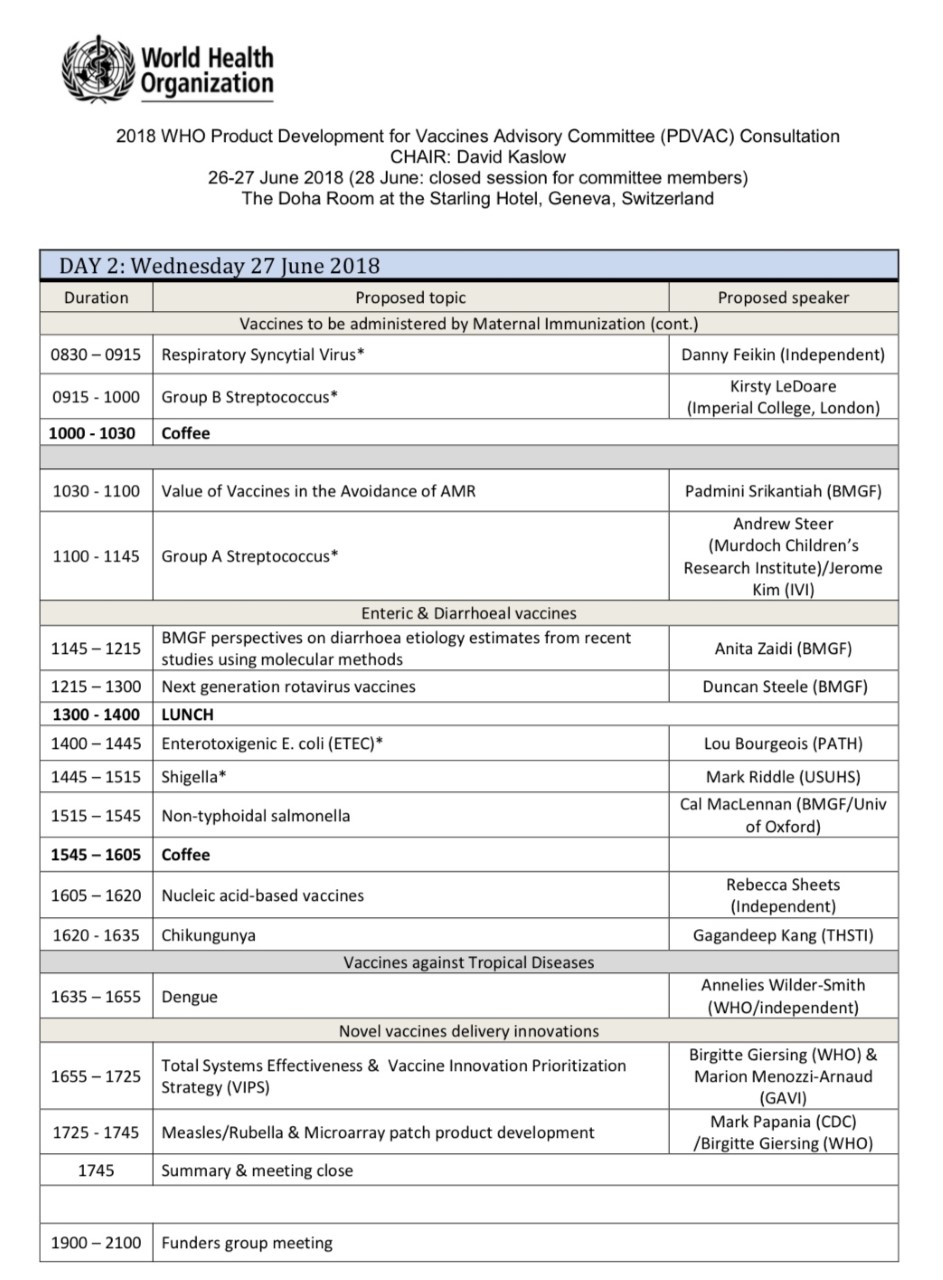
WHO-PDVAC 5th Annual Meeting
Date: June 26-27, 2018
Type of Event: WHO-PDVAC
WHO’s Product Development for Vaccines Advisory Committee (PDVAC) was convened for its 5th annual meeting. This was building on previous PDVAC meetings in 2015 and 2017 that put Strep A vaccines on their agenda. Over two days, progress was discussed in vaccine and monoclonal antibody development for the 10 previously prioritized pathogen areas including Streptococcus A vaccines. Several cross-cutting topics were considered, such as the potential role of vaccines in addressing antimicrobial resistance (AMR) and two new vaccine product development initiatives, namely Total Systems Effectiveness (TSE) and the Vaccine Innovation Prioritization Strategy (VIPS), were presented.
SAVAC Kick-off, Perth, March 7-8, 2019
Date: March 7-8, 2019
Type of Event: Kick-off meeting
Core members of the SAVAC attended the kick-off meeting to cover various issues on accelerating the development of a Group A streptococcus (GAS) Vaccine. The kick-off meeting identified the activities to be organized in each of its six pillars, which entailed Epidemiology, the burden of disease, the business case with landscape analysis in the literature review, traditional investment case, and encompassing health, social, and economic benefits. Among one its general considerations, all acknowledged that SAVAC would work together and with WHO/IVR, and strengthen the interaction between partners and stakeholders to streamline the implementation phase. The outreaching objectives were to address preliminary work plans and to justify the development of a vaccine for GAS through a comprehensive WHO PHVP of which the business case and the global health investment case.
SAVAC in Cape Town, March 13-15, 2019
Date: March 13-15, 2019
Type of Event: The Inaugural PROTEA Workshop, Partnerships For Children With Heart Disease In Africa
With the aim of fostering ground-breaking, bold, scalable research ideas for RHD for significant global impact, the meeting was held to support interdisciplinary partnerships for children with heart disease in Africa. Sponsored by the University of Manchester, the University of Cape Town, Medical Research Council (MRC), and Children’s’ Heart Disease Research Unit, the meeting highlighted four distinct themes: (1) bringing together and further train colleagues from South Africa and around the World to focus on novel research ideas to impact and change the lives of those living with RHD (2) providing feedback and further training into Echocardiography as we demonstrate how research can impact clinical practice (3) Congenital heart disease, diagnosis, follow-up care, surgery, and genetics. (4) RHD Prevention with plenary presentations and Think Thank sessions aiming at identifying various gaps in diagnosis, disease burden, care & treatment, and prevention including vaccines.

Prof. Andrew Steer, Co-Chair of SAVAC, delivers a lecture on “Development of Strep A Vaccine” at IVI
Date: September 02-06, 2019
Type of Event: IVI International Vaccinology Course
The Co-Chair of SAVAC, Andrew Steer, was invited to deliver a lecture on ‘Development of Strep A Vaccine' during IVI's 19th International Course of Vaccinology 2019. In addition to 134 trainees from 49 countries, the five-day course brought together 41 individual experts from academia, government, industry, and non-governmental organizations, thereby establishing a comprehensive overview of vaccinology with a focus on practicality.

Prof. Andrew Steer, IVI International Vaccinology Course, September 2-6, 2019.
On September 4, 2019, Prof. Andrew Steer covered a wide spectrum on Strep A vaccine development from discovery to delivery, to help increase developing nations' capacity in vaccine research and immunization.
“The Group A streptococcus (GAS), Streptococcus pyogenes, is the biggest infectious killer that no one has heard of. Infection with GAS causes 500,000 annual deaths,” he said. “The enormity of this problem stands in contrast with resources available to develop a vaccine for GAS. GAS vaccine funding as well as develop documents justifying investment in GAS vaccines at business and policy levels, will fill a critical need.”
